Emulsifiers in baking
In this post, we are revisiting additives, specifically one of the most commonly used ones in baking. However, the function of these additives in baking is quite different from what is typically portrayed in most additive reference materials, so pay close attention.
An emulsifier is a chemical substance with a hydrophilic (water-friendly) part and a lipophilic (fat-friendly) part. The primary purpose of these substances lies in their ability to facilitate the creation of emulsions, or mixtures of substances that are not typically miscible under normal conditions. A typical example is the mixture of water and oil. We all know that when we mix these two substances, they tend to separate quickly. If we apply mechanical energy (such as stirring), we can dissolve the oil into water in the form of tiny droplets, but they tend to recombine and separate from the aqueous phase. This separation occurs due to the high repulsive forces in the region between the two phases (surface tension) and the natural tendency toward equilibrium or forms with less tension. When an emulsifier is added to a mixture of water and oil, it positions itself between both phases, with the hydrophilic part facing the aqueous phase and the lipophilic part facing the oily phase, reducing surface tension. The more droplets there are, and the smaller their size, the larger the surface area, and the greater the need for emulsifiers.
So far, this explanation aligns with what you will find in most books and references on additives. However, in baking, the function of emulsifiers is quite different. On the contrary, the use of emulsifiers in baking is based on their ability to strengthen dough and reduce bread staling during storage. Emulsifiers are also commonly used in whipped dough due to their capacity to enhance foam formation and stability. One of the products in which emulsifiers can fulfill their well-known role is creams, especially those that contain a mixture of water and oil.
The use of emulsifiers in baking dates back to the mid-20th century. In the 1930s, fats mixed with emulsifiers were already commercialized, allowing for the reduction of fat content in certain products by improving fat functionality. However, it was in the 1950s and 1960s that the use of emulsifiers in baking, particularly in products like sandwich bread, became widespread due to their anti-staling effects. Nowadays, emulsifiers are one of the most used additives, along with ascorbic acid, in most dough conditioners.
Dough Strengtheners
Emulsifiers can have a dough-strengthening effect. This effect is perhaps the primary reason for using emulsifiers in baking today and is based on their interaction with gluten. The fundamental principles of this interaction are not well understood, and various theories have been proposed, from bonds between certain hydrophobic parts of proteins and hydrophobic regions of emulsifiers to bonds based on electrical charge, or other types of bonds. The most widespread theory is based on charge interactions, as not all emulsifiers have this strengthening effect, with anionic emulsifiers (with a negative charge on their lipophilic part) being the ones displaying this effect.
Regardless of the mechanism, the strengthening effect of emulsifiers is distinct from other dough strengtheners like oxidizers. During kneading, doughs with emulsifiers can tolerate more water, although this increase is minimal and not observed with all emulsifiers. In some cases, the dough may require slightly more kneading, but what seems clear is that doughs are more tolerant of excessive kneading. As for the tenacity and extensibility of doughs, the effects are minor and vary depending on the emulsifier, but there is a reduction in dough stickiness, making it easier to handle. Perhaps the most significant effect of emulsifiers is the improvement in gas retention during fermentation and the volume of bread.
Unlike other products, the effects of emulsifiers are hardly noticeable in the initial phases of fermentation, making them less perceptible in lightly fermented bread. However, in the final stages of fermentation, when the dough is weaker, emulsifiers come into play. During this period, a slight bump between trays in production lines or abrupt movement, or excessive fermentation, can cause dough to collapse. Emulsifiers prevent this collapse by reducing problems and allowing for excessive fermentation without dough collapse. In general, emulsifiers provide process tolerance, reducing the issues associated with excessive kneading or fermentation. These effects of emulsifiers are more evident in doughs made with weak flours and those that incorporate ingredients that weaken the dough, such as bran.
As for breads with strengthening emulsifiers, they have a larger volume (especially with longer fermentation), softer crumbs (partly due to the increased volume), and less crumbly texture, making them highly appreciated for making sandwich bread.
As we mentioned, not all emulsifiers have a strengthening effect, and the most effective one is DATEM (E-472e). This function is also performed by SSL (E-481i) and CSL (E-482), and in some countries, the use of polysorbates (E-432-436) or sucrose esters (E-473) is common.
Emulsifiers can also enhance the specific volume of bread through another mechanism. During baking, especially in the early stages, several phenomena increase dough volume. These phenomena include gas expansion due to heat and, primarily, the final phase of fermentation. However, dough expansion ends when starch gelatinizes, increasing dough consistency and making it less flexible, thereby losing its ability to expand. Anything that increases the gelatinization temperature of starch, thus delaying this phenomenon during baking, allows for more dough expansion. Oils and fats, as well as emulsifiers, have this effect. However, unlike the strengthening effect, this effect is not related to the emulsifier’s electrical charge, and emulsifiers without a strengthening effect, like monoglycerides (which we will discuss shortly), also exhibit this action.
Reduction of Bread Staling
As we all know, bread is a highly perishable product and one of the leading contributors to food waste, both in distribution and households. Therefore, one of the main objectives of manufacturers has been to extend the shelf life of bread. The deterioration of bread can result from various causes, with the dominance of one or the other cause depending on the type of bread, which will be discussed in another section. However, there is a common phenomenon in most bread, which is its tendency to harden.
Bread staling occurs due to two fundamental reasons: dehydration and starch retrogradation. Once baked, bread has a moist inner part and a drier outer part. However, in nature, everything tends to equalize unless there is something preventing it, and the moisture in the crumb tends to migrate to the crust to balance this inequality. This phenomenon is responsible for the progressive drying of the crumb and the loss of crispness in the crust, making it chewy. In bread with a moist and soft crust, such as sandwich bread, this phenomenon is hardly noticeable. In cases where the atmosphere is dry, the moisture in the crust will evaporate into the air, reducing crust chewiness. However, if bread is stored in a way that retains moisture, like plastic-wrapped sandwich loaves, you may observe a hardening of the crumb over time, which is not due to dehydration. This hardening is more pronounced at lower storage temperatures. This is why it is not recommended to store sandwich bread in the refrigerator, even though this practice reduces mold growth. This hardening is caused by starch retrogradation.
Retrogradation involves the rearrangement of starch after it has been gelatinized, resulting in more rigid and harder structures. Starch is composed of amylose and amylopectin. Although many references claim that amylose is responsible for starch retrogradation, this is only partially true. Amylose retrogrades quickly, leading to the texture of gelatinized starch after rapid cooling. Therefore, amylose retrogradation is responsible for the crumb’s texture, which progressively hardens after baking or the texture of cooked rice, and whether it is loose or sticky. On the other hand, amylopectin retrogrades more slowly and seems to be responsible for bread hardening during storage, with this reaction being faster at lower temperatures.
Some emulsifiers can interact with starch, reducing the rate of retrogradation reactions and, consequently, crumb hardening due to this process. Oils and fats also have this effect, and they are responsible for products with high fat content hardening less than those without fat. In general, emulsifiers capable of delaying retrogradation are the same ones that increase the gelatinization temperature of starch, and thus they can also increase bread volume through greater expansion during baking. Monoglycerides and SSL are emulsifiers known for having this effect, while DATEM has a much weaker effect. Monoglycerides are generally the preferred emulsifiers for reducing staling phenomena, although SSL can be used when a mixed effect is desired. In the classification of additives, the E-471 number is assigned to mixtures of mono- and diglycerides, but diglycerides are not effective in reducing bread staling. Therefore, the products used for this purpose must be purified to reduce the presence of diglycerides. A dose of 0.75-1% based on flour is usually sufficient to notice this effect, while higher amounts have little impact.
The use of these emulsifiers is crucial in the production of sandwich bread and long-shelf-life bread, where the main cause of staling is starch retrogradation, and delaying these phenomena can extend the shelf life by several days. However, in lean formula breads with crisp crusts, the effect of monoglycerides is usually not as noticeable or as important, as these breads lose their characteristic texture for other reasons and do so quickly (within one or two days).
As Foam-Forming Agents
The third most important application of emulsifiers in the production of cereal-based baked goods is not focused on bread but rather on batter-based products such as cakes, muffins, tea pastries, and similar items. In most of these batters, a gluten network does not form, making the interaction of emulsifiers with wheat proteins less significant. However, their effect on starch may be of interest, as it delays starch gelatinization during baking, allowing for greater expansion and can slow retrogradation, reducing hardening and extending shelf life. This effect is more pronounced in batters with low fat content, as fats have a similar effect. The most important effect is related to their foaming ability, both in retaining air during mixing and stabilizing this air inside the batters.
In cakes and similar products, it’s crucial to trap air inside the batter and to retain the trapped air and that generated during baking. During mixing, small air bubbles are incorporated. During baking, the incorporated air expands, and new gases are generated by the action of leavening agents. These gases will be trapped in the existing bubbles (no new bubbles are formed), increasing their size. The key to making a good cake is to incorporate the smallest air bubbles possible, as larger bubbles are more mobile and can merge, forming even larger bubbles that can escape to the surface of the batter and the atmosphere. Other factors, such as batter viscosity, also play a role, but internal air dispersion is usually the most critical factor.
In these products, where there is air dispersion inside an aqueous batter, emulsifiers can help stabilize this foam by positioning themselves at the interface between water and air. The hydrophilic portion of the emulsifier would be oriented toward the aqueous batter, while the hydrophobic portion would be oriented inside the bubble.
In general, two types of cakes can be distinguished. One group includes products like muffins, pound cakes, layer cakes or similar items, made with oil or fats in their formulation, along with leavening agents. These cakes are moist and can be eaten without fillings or toppings. On the other hand, there are sponge or chiffon cakes, which are much airier, have little fat in their composition, and usually do not contain leavening agents. These cakes are drier and are usually consumed with some filling (cream, whipped cream, jams, chocolate, etc.) or toppings.
In the first type of cake, the air bubbles are surrounded by a thin layer of oil that stabilizes them, preventing coalescence (the joining of several bubbles). The number of bubbles is not very high, so leavening agents are used during baking to expand these bubbles further. In general, emulsifiers are not necessary for making these cakes, and in fact, they can be made at home without these additives. However, the use of emulsifiers can be very beneficial when making cakes with low oil or fat content since a decrease in oil reduces its ability to stabilize bubbles, a role that emulsifiers can perform. Emulsifiers can also be helpful when attempting to achieve a finer crumb structure using oils (fats generate finer and more uniform crumb structures), although in Spain, open crumb structures are typical of these products. Typically, to improve the crumb structure of these products, ingredients are mixed in phases. In cakes with fats, they are mixed with sugars (creaming) to trap gases, and then the remaining ingredients are incorporated. Emulsifiers can also help if you want to create a process where all the ingredients are mixed together.
In the second type of cake, the air bubbles are stabilized by egg proteins, which have excellent foaming properties. The amount of incorporated air is much higher than in oil-based cakes, and the simple expansion of these gases during baking due to temperature increases leads to a significant volume increase, without the need for leavening agents. However, the presence of fats can reduce the effectiveness of egg proteins in performing this role. This is why, at home, egg whites are carefully separated from the yolks and whipped separately, incorporating a large amount of air. In the industry, this practice is usually not feasible, so incorporating and stabilizing air bubbles is more complex. To avoid these issues, emulsifiers can be used, as they facilitate this process when the ingredients are mixed together.

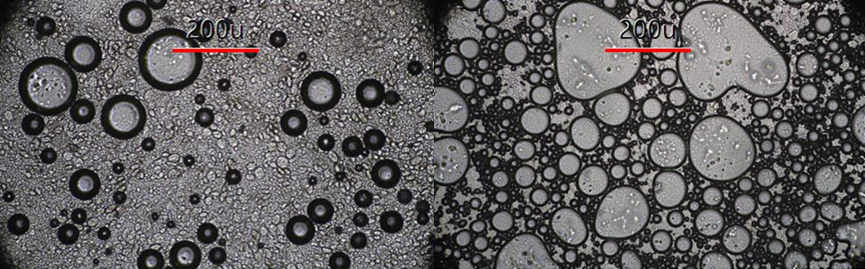
Figure 1: Microscope images of fat-based (left) and egg-based (right) batters.
In general, we can state that emulsifiers are not necessary for making cakes, but they can be helpful when deviating from traditional recipes and processes, either for convenience or nutritional reasons. Emulsifiers that crystallize in the α form are much more effective for this purpose. Therefore, lactyl or acetyl esters of monoglycerides, or propylene glycol esters of fatty acids, which tend to crystallize in the α form, are preferred. Monoglycerides in the α crystal form, Polysorbate 60, or certain sucrose esters can also be used.
For more information,
• Stauffer, C.E. (1999) Emulsifiers. Eagan Press. St Paul, MN (USA)
• Stauffer, C.E. (1990) Functional additives for bakery foods. Van Nostrand Reinhold. New York (USA)